The EpiOral™ and
EpiGingival™ Tissue Models
주문은 최소 1개월전에 하셔야 합니다.
- Buccal and Gingival Phenotypes
- 3-Dimensional, Highly Differentiated
- Human Skin-Like Structure
- Based on Normal Human Cells
- In Vivo-Like Lipid Profile
- Completely Serum-Free Media System
- Highly Reproducible
- Easily Handled Cell Culture Inserts
- Quantifiable, Objective Endpoints
- Ideal for Irritation, Oral Pathology, and Basic Studies
- Cost Effective Alternative to Animal and Clinical Testing
To enable in vitro study of irritation, oral pathologies, and basic oral cavity phenomena, MatTek has developed the EpiOral series of tissue models. MatTek's EpiOral and EpiGingival tissues consist of normal, human-derived epithelial cells. The cells have been cultured to form multilayered, highly differentiated models of the human buccal (EpiOral) and gingival (EpiGingival) phenotypes. The tissues, which are cultured on specially prepared cell culture inserts using serum free medium, attain levels of differentiation on the cutting edge of in vitro cell culture technology. Morphologically, the tissue models closely parallel native human tissues, thus providing a useful in vitro means to assess irritancy, disease and other basic oral biology phenomena.
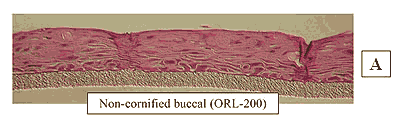
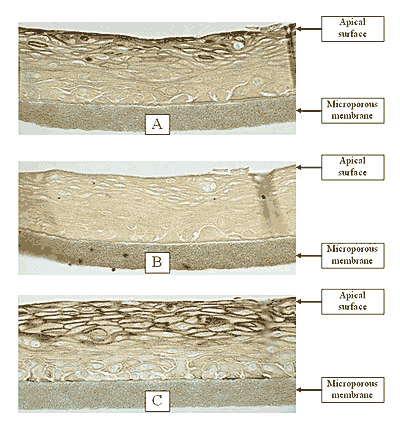
Figure 1: HISTOLOGY: EpiOral™(buccal phenotype)
Formalin Fixed, Paraffin Embedded, H&E Stained
(Click on photo to see larger image.)
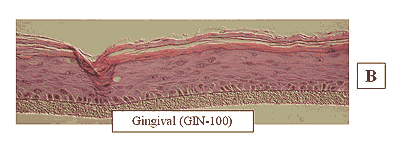
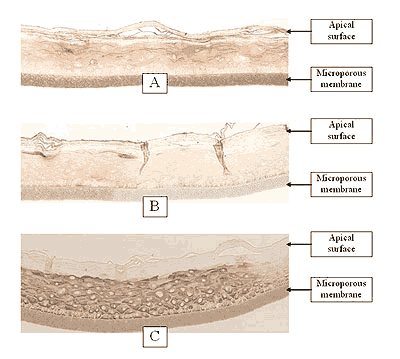
Figure 2: HISTOLOGY EpiGingival™(gingival phenotype)
Formalin Fixed, Paraffin Embedded, H&E Stained
(Click on photo to see larger image.)
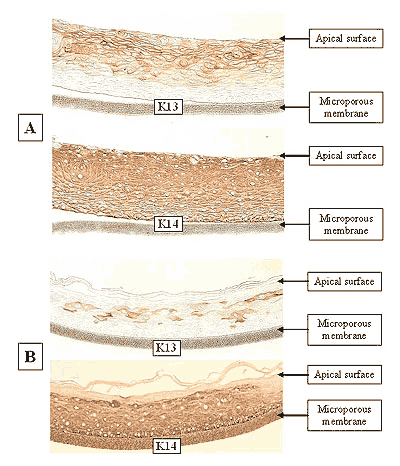
The EpiOral and EpiGingival tissue models exhibit in vivo-like morphological and growth characteristics which are uniform and highly reproducible. EpiOral is a multilayered tissue consisting of an organized basal layer and multiple non-cornified layers analogous to native human buccal tissue (Figure 1). The EpiGingival tissue is also multilayered except that the apical layers are cornified, similar to in vivo gingival tissue (Figure 2). Both tissues express cytokeratin K13 in the all layers except the most apical ones and weakly express cytokeratin K14 in the upper layers of the tissue (Figure 3). The tissues also produce the naturally occurring antimicrobial peptides called human beta defensins (HBD). As shown in Figure 4, the ORL-200 constitutively expresses HBD-1 and HBD-3 but not HBD-2. The GIN-100 tissue weakly expresses HBD-3 in all layers except the stratum corneum, expresses HBD-1 weakly only in the apical layers, and does not express HBD-2 (Figure 5). Lipid analysis of the tissues revealed the presence of only Ceramide 2 (Ceramide NS) in EpiOral and Ceramides 1, 2, and 3 (also referred to as Ceramides EOS, NS, and EOHP/NP, respectively) in the EpiGingival tissue, overlapping normal lipid profiles of in vivo tissue.
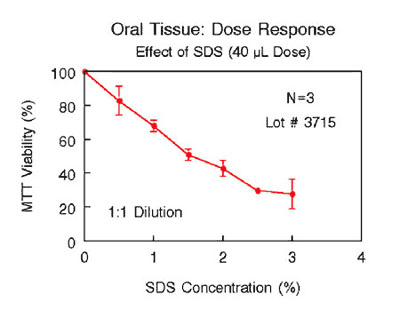
Various industrial and toxicology laboratories are actively seeking alternatives to expensive clinical or whole animal testing. Oral care, personal care, and pharmaceutical companies have initiated in vitro toxicology testing to evaluate their raw materials and final product formulations. Companies utilize antibiotic/antifungal free EpiOral tissue (ORL-200-AFAB) to grow various commensal and pathogenic bacteria in order to study their effects on the oral tissues. Figure 6 shows the effects of SLS, a common active ingredient of dentifrices, on the viability of ORL-200 tissue. As shown therein, a linear relationship exists between tissue viability and the SLS concentration. Such an assay can be used to predict oral irritancy of oral care products. A growing body of data indicates that EpiOral and EpiGingival effectively provide an inexpensive, non-animal means to assess oral irritation, toxicology, and pathology related issues.
The protocols for using EpiOral and EpiGingival are clear and straightforward. The tissue have been utilized with several common tests of cytotoxicity and irritancy, including MTT and IL-1α. mRNA can easily be harvested to analyze gene expression and the culture medium can be analyzed using ELISA assays to measure cytokine release. Technicians find the rigid substrate design of EpiOral and EpiGingival easy to handle and manipulate in routine repetitive testing environments and scientists find that they are able to perform discriminating tests due to low background interference.
 |
|
Figure 3: Immuno-staining of: A) EpiOral and B) Gingival tissue for cytokeratin K13 and K14. Negative control (no antibody) tissue did not stain, similar to basal layer in EpiOral K13 section in A).
(Click on photo to see larger image.) |
 |
|
Figure 4: Immuno-staining of the reconstructed ORL-200 tissue for: A) human defensin beta (HBD)-1, B) HBD-2, and C) HBD-3. The negative control slide without the primary antibody is identical to hBD-2 slide. Objective = 20X.
(Click on photo to see larger image.) |
 |
|
Figure 5: Immuno-staining of the reconstructed GIN-100 tissue for: A) human defensin beta (HBD)-1, B) HBD-2, and C) HBD-3. The negative control slide without the primary antibody is identical to hBD-2 slide. Objective = 20X.
(Click on photo to see larger image.) |
| Lipid Species |
Buccal (n=14) |
Std. Dev. |
ORL-100 |
Gingival (n=14) |
Std. Dev. |
GIN-100 |
| PL |
76.3 |
8.9 |
93.1 |
78.7 |
6.7 |
86.4 |
| GSL |
23.0 |
7.8 |
3.7 |
16.9 |
6.0 |
3.9 |
| AGC |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
| CER AH |
0.0 |
0.0 |
0.00 |
0.79 |
0.33 |
0.00 |
| CER AP |
0.0 |
0.0 |
0.00 |
0.0 |
0.0 |
0.00 |
| CER NH |
0.0 |
0.0 |
0.00 |
0.88 |
0.43 |
0.00 |
| CER AS |
0.0 |
0.0 |
0.00 |
0.0 |
0.0 |
0.00 |
| EOHP/ NP |
0.0 |
0.0 |
0.00 |
0.47 |
0.16 |
0.78 |
| CER NS |
0.72 |
0.32 |
2.4 |
1.7 |
0.52 |
5.8 |
Table 1: Lipid analysis of in vivo and in vitro tissues. The in vivo buccal and gingival values are averages for 14 tissues samples. The in vitro tissue values are from a single lot.
Key: PL = Phospholipid, GSL = Glucosphingolipid, AGC = Acyl glucosylated ceramide, CER AH = ceramide 7, CER AP = ceramide 6, CER NH = ceramide 5, CER AS = ceramide 4, CER EOHP/NP = ceramide 3, CER NS = ceramide 2, CER EOS = ceramide 1 (1)
(1) Ponec M, Weerheim A, Lankhorst P, Wertz PW: New acylceramide in native and reconstructed epidermis. J Invest Dermatol 120:581-588, 2003.
 |
|
Figure 6: Effect of SDS solutions (40 μL) on ORL-200 tissue viability following exposure for 1 hour. SDS concentrations were chosen to be in the range normally present in toothpastes (0.0 – 3.0%). |
Initial SDS
Conc. (%) |
ET-50 (min) |
| ORL-200** |
GIN-100** |
| 0.75 |
>240 |
179 |
| 1.50 |
79 |
116 |
| 2.25 |
41 |
88 |
| 3.00 |
36 |
--- |
** ORL-200: soln. diluted 1:1. Dose = 40µL
** GIN-100: neat soln. Dose = 100µL |
Table 2: Effect of sodium dodecyl sulfate (SDS) on ORL-200 and GIN-100 tissue. The tissue viability was determined using the MTT assay and the exposure time that reduces the tissue viability to 50% (ET-50) for each SDS solution was determined as follows:
ORL-200: Solution diluted 1:1 with ultrapure water, 40 µL of diluted solution applied.
GIN-100: 100 µL of neat solution applied.