|
The EpiDermFT™ Skin Model
주문은 최소 1개월전에 하셔야합니다.
EpiDermFT-Specific Features:
- Human 3-D Skin-Like Structure – Epidermis & Dermis
* Normal, Human-Derived Epidermal Keratinocytes
* Normal, Human-Derived Dermal Fibroblasts
* In Vivo-Like Lipid Profile
* All In Vivo Cermides Present
* Broad-Narrow-Broad Lamellar Spacing
- Well Developed Basement Membrane
- Ideal for Keratin Gene Expression, Photo-Damage, Wound Healing, Keratinocyte/Fibroblast Signaling and Integrin Signaling Studies
General MatTek Tissue Features:
- Unsurpassed Long-Term Tissue Reproducibility -
Lot-to-Lot, Year-to-Year
- 3-Dimensional, Highly Differentiated Tissues
- Metabolically, Mitotically Active Tissues
- Produced from Normal (Non-Transformed) Human Cells - Ideal for Genomics Studies
- Produced in Easily Handled Cell Culture Inserts
- Grown in Completely Serum-Free Media System
- Quantifiable, Objective Test Endpoints
- Cost Effective Alternative to Animal and Human Clinical Testing
- List of Contract Testing Labs Qualified to Run MatTek Tissue-Based Tests Available
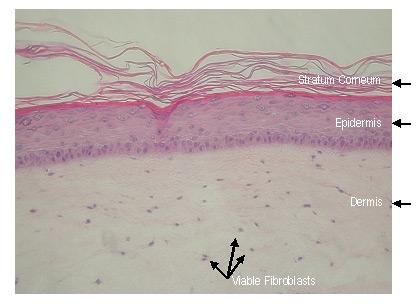
The EpiDermFT Full Thickness Skin Model
To enable in vitro study of dermal phenomena in which fibroblast-keratinocyte cell interactions (paracrine signaling) are important, MatTek has developed EpiDermFT, a full thickness skin model. MatTek's EpiDermFT System consists of normal, human-derived epidermal keratinocytes (NHEK) and normal, human-derived dermal Fibroblasts (NHFB) which have been cultured to form a multilayered, highly differentiated model of the human dermis and epidermis. The NHEK and NHFB, which are cultured on specially prepared cell culture inserts using serum free medium, attain levels of differentiation on the cutting edge of in vitro skin technology. Ultrastructurally, the EpiDermFT Skin Model closely parallels human skin, thus providing a useful in vitro means to assess dermal irritancy and toxicology.
 |
|
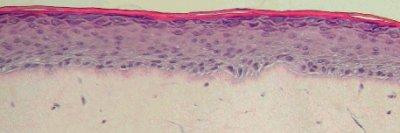
Figure 1: Histology of EpiDermFT. H&E Stained paraffin section reveals epidermis containing basal, spinous, granular keratinocytes and stratum corneum. Dermis contains numerous viable fibroblasts. 400X)
|
The EpiDermFT Full Thickness Skin Model exhibits in vivo-like morphological and growth characteristics which are uniform and highly reproducible.
EpiDermFT consists of organized basal, spinous, granular, and cornified epidermal layers analogous to those found in vivo. The dermal compartment is composed of a collagen matrix containing viable normal human dermal fibroblasts (NHDF).
EpiDermFT is mitotically and metabolically active. Markers of mature epidermis-specific differentiation such as pro-filaggrin, the K1/K10 cytokeratin pair, involucrin, and type I epidermal transglutaminase have been localized in the model.
Ultrastructural analysis has revealed the presence of keratohyalin granules, tonofilament bundles, desmosomes, and a multi-layered stratum corneum containing intercellular lamellar lipid layers arranged in patterns characteristic of in vivo epidermis.
A well-developed basement membrane is present at the dermal/epidermal junction. Hemidesomosomes, lamina lucida, lamina densa and anchoring fibril structures are evident by transmission electron microscopy. Immunohistochemical analysis shows the presence of basement membrane structural and signaling proteins including collagen IV, Laminin, collagen VII and integrin α6.
Various industrial and toxicology laboratories are actively seeking alternatives to whole animal testing. Cosmetic, household product, pharmaceutical and petrochemical companies have initiated in vitro toxicology testing to evaluate their raw materials and final product formulations. A growing body of data indicates that EpiDermFT effectively provides a non-animal means to assess dermal toxicology and skin research issues.
The protocols for using the EpiDermFT System are clear and straightforward. EpiDermFT has been utilized with several common tests of cytotoxicity and irritancy, including MTT, IL-la, PGE2, MMP-1 and apoptosis. Technicians find EpiDermFT's rigid substrate design easier to handle in routine repetitive testing environments and scientists find that they are able to perform discriminating tests due to low background interference.
|
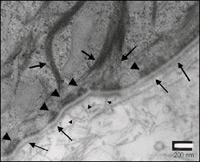
Figure 2: TEM of EpiDerm-FT Basement Membrane. Higher magnification TEM shows finer detail of basement membrane structure:
Lamina Densa (------------>)
Hemidesmosome (>)
Anchoring fibril (->)
Tonofilament (------>)
Note the anchoring fibrils beneath the lamina densa and tonofilament association with hemidesmosomes.
|
 |
 |
|
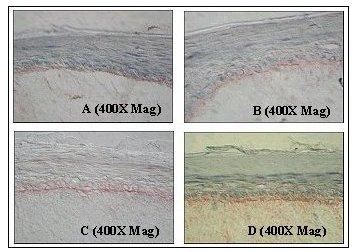
Figure 3: Immunohistochemical Analysis of Basement Membrane Structural Proteins in EpiDermFT.
Frozen sections of EpiDerm-FT were immunostained for: A) Collagen Type IV (component of lamina densa); B) Laminin 5 (component of lamina lucida); C) Collagen Type VII (component of anchoring fibril); D) Integrin a-6 (component of hemidesmosome). Specific staining of the indicated protein is displayed as a red band localized at the dermal/epidermal junction.
|
 |
|
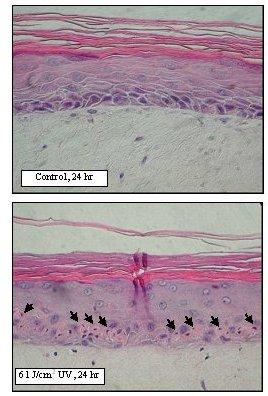
Figure 4. Sunburn cell formation in EpiDerm-FT following solar UV-irradiation. H&E stained paraffin sections were prepared from EpiDerm-FT 24 hr after irradiation. Sunburn cells are indicated by arrows.
|
The EpiDermFT™ Technical Specifications
I. Ordering
- New Orders: MatTek Scientists will consult on your application at no additional charge. It is STRONGLY RECOMMENDED that you have this consultation BEFORE placing your first order to help ensure that all media and accessories needed to perform your application are included in the initial order.
- ALL Tissue Orders: Please allow 3-4 weeks from order date to delivery of tissues. Please contact MatTek Customer Service for additional details.
II. Cells
- Type: Normal human epidermal keratinocytes (NHEK); Normal human dermal fibroblasts (NHDF).
- Genetic make-up: Single donor.
- Derived from: Neonatal-foreskin tissue (NHEK); Neonatal skin (NHDF)
- Alternatives: NHEK from adult breast skin; NHDF from adult skin.
- Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma.
III. Medium
- Base medium: Dulbecco's Modified Eagle's Medium (DMEM).
- Growth factors/hormones: Epidermal growth factor, insulin, hydrocortisone and other proprietary stimulators of epidermal differentiation.
- Serum: None.
- Antibiotics: Gentamicin 5 µg/ml (10% of normal gentamicin level).
- Anti-fungal agent: Amphotericin B 0.25 µg/ml.
- pH Indicator: Phenol red.
- Other additives: Lipid precursors used to enhance epidermal barrier formation (proprietary).
- Alternatives: Phenol red-free (EFT-400-PRF), antibiotic-free (EFT-400-ABF), anti-fungal-free (EFT-400-AFF), or hydrocortisone-free medium and tissue (EFT-400-HCF) are available. Agents are removed at least 3 days prior to shipment.
- Assay/Maintenance medium: EFT-400-ASY is utilized for assays; EFT-400-MM is used for term maintenance of the EFT-400 tissues.
IV. Tissue
- Kit: Standard EpiDermFT kit (EFT-400, formerly EFT-200) consists of 24 tissues. (Tissue "kits" contain tissues, a small amount of culture medium, and plasticware; contact MatTek for specific kit contents.)
- Substrate: Costar Snapwell™ single well tissue culture plate inserts are used. Pore size = 0.4 μm, Diameter = 1.2 cm. Surface area = 1.0 cm2.
- Culture: At air liquid interface.
- Histology: 8-12 cell layers plus stratum corneum (basal, spinous, and granular layers).
- Lot numbers: Tissue lots produced by each technician for each week are assigned a specific lot number. Typically, there are multiple lot numbers for any given week's tissue production. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical in regards to cells, medium, handling, culture conditions, etc.
- Shipment: At 4°C on medium-supplemented, agarose gels in 24-well plate.
- Shipment day: Every Monday. Shipment on Thursday also possible upon special request.
- Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Thursday depending on location.
- Shelf life: Including time in transit, tissues may be stored at 4°C for up to 6 days prior to use. However, extended storage periods are not recommended unless necessary. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Tuesday afternoon or following overnight storage at 4°C (Wednesday morning).
- Length of experiments: Cultures can be continued for up to 2 weeks with good retention of normal epidermal morphology. Cultures must be fed every other day with 5.0 ml of EFT-400 medium.
- Alternative tissues:
EFT-400-ABF: Antibiotic-free tissue. For last 3 days of culture, gentamicin is omitted from culture medium. Customer also receives EFT-400-ASY-AFAB medium.
EFT-400-AFF: Anti-fungal-free tissue. For last 3 days of culture, Amphotericin is omitted from culture medium. Customer also receives EFT-400-ASY-AFAB medium.
EFT-400-AFAB: Antibiotic, anti-fungal-free tissue. For last 3 days of culture, gentamicin and Amphotericin are omitted from culture medium. Customer also receives EFT-400-ASY-AFAB medium.
EPFT-400-FRZN: EFT-400 tissue that has been subjected to 3 freeze/thaw cycles. Designed to serve as control for MTT auto-reduction test.
EFT-412: Same as EFT-400 except 12 cultures instead of 24
EFT-300: Same as EFT-400 except grown in 9 mm ID Millipore Millicell™ Single Well Tissue Culture Plate Inserts (24 tissues)
EFT-306: Same as EFT-300 except 6 tissues, each tissue grown in a 22 mm ID Millipore Millicell™ Single Well Tissue Culture Plate Insert.
V. Quality Control and Sterility
- Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment.
- End-use testing: Tissues are exposed to 1% Triton X-100 for 8, 12, 16 and 24 hours. The time of exposure required to reduce the tissue viability (ET-50) using the MTT viability assay is determined (See MatTek EpiDermFT use protocol) for each lot of tissue. ET-50's generally fall within the range of 6.5-9.5 hours. ET-50's in customers' lab may differ slightly from the MatTek results.
- Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination.
- Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service.
- Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because our QC and sterility testing is done post-shipment, notification will be made as soon as possible (Under normal circumstances, ET-50 failures will be notified by Wednesday 5 p.m.; sterility failures will be notified within 8 days of shipment).
Guide to In Vitro Tissue Model Basics
|