Feminine Hygiene ( EpiVaginal )
The Model
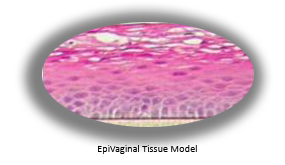
To facilitate the study of vaginal-ectocervical (VEC) toxicity, pathologies, and basic mucosal phenomena, MatTek has developed the EpiVaginal series of tissue models. EpiVaginal tissues are based on normal, human-derived VEC epithelial and dendritic cells (DC). All VEC tissues are cultured on specially prepared cell culture inserts and are multilayered and highly differentiated. The tissues closely parallel native human tissues, thus providing a useful in vitro means to assess toxicity, sexually transmitted infections, and other basic vaginal phenomena. For more information on EpiVaginal, click here.
Technical References

699. ASSESSMENT OF AN IN VITRO HUMAN VAGINAL EPITHELIAL MODEL FOR PREDICTING VAGINAL IRRITATION. Evans1, E.B., Priston1, R., Willoughby2, J.A. Sr., Wagner2, H.N. and McKim2, J.M. Jr. 1Kimberly-Clark Corporation, Roswell, GA. 2Ceetox, Inc., Kalamazoo, MI. Presented at SOT 2012, Abstract 1816.
656. EPIVAGINAL TISSUE MODEL FOR PRECLINICAL SCREENING OF SINGLE OR REPEAT EXPOSURE TO VAGINALLY APPLIED CHEMICALS/FORMULATIONS. Ayehunie, S., Cannon, C., LaRosa, K., Landry, T., Wang, A., and Klausner, M. MatTek Corporation, Ashland, MA, USA. Presented at SOT 2011, Abstract #1049
639. ASSESSMENT OF ORGANOTYPIC EPIVAGINALTM TISSUE MODEL TO SCREEN IRRITATION POTENTIAL OF CHEMICALS. Ayehunie, S., Kaluzhny, Y., Cannon, C., LaRosa, K., Klausner, M. MatTek Corporation, Ashland, MA. Presented at 49th Annual SOT Meeting, Salt Lake City, Utah (2010)
611. IN VITRO ASSESSMENT OF PERSONAL CARE PRODUCTS FOR VAGINAL IRRITATION USING 3D TISSUE CONSTRUCTS. Evans1, E., Priston1, R., Inglis2, H., Barnes2, N., Raabe2, H., Costin2, G.-E. 1The Kimberly-Clark Corporation, Roswell, GA, USA. 2Institute for In Vitro Sciences, Inc. (IIVS), Gaithersburg, MD, USA. Presented at Society of Toxicology Meeting, Salt Lake City, UT March 2010.
577. SAFETY TESTING OF SILICONE ELASTOMER MATRIX VAGINAL RINGS CONTAINING UC781 AND TWEEN 80 USING EPIVAGINAL(VEC-606) TISSUES. McConville1, C., Woolfson1, A.D., Elgendy2, H., Friend2, D., Malcolm1, R.K. 1School of Pharmacy, Queens University of Belfast, Belfast, UK 2CONRAD, Arlington, VA, USA. Presented at Microbicide 2010 Meeting, Pittsburgh, PA
426. IRRITATION TESTING OF CONTRACEPTIVE AND FEMININE-CARE PRODUCTS USING EPIVAGINAL, AN IN VITRO HUMAN VAGINAL-ECTOCERVICAL TISSUE MODEL. Ayehunie, S., Hayden, P., Cannon, C., Lamore, S., Klausner, M., Sheasgreen, J. MatTek Corporation, Ashland, MA. Presented at EUROTOX 2006, Cavtat and Dubrovnik, Croatia, September 20-24, (2006).