Drug Delivery ( EpiOral )
The Model
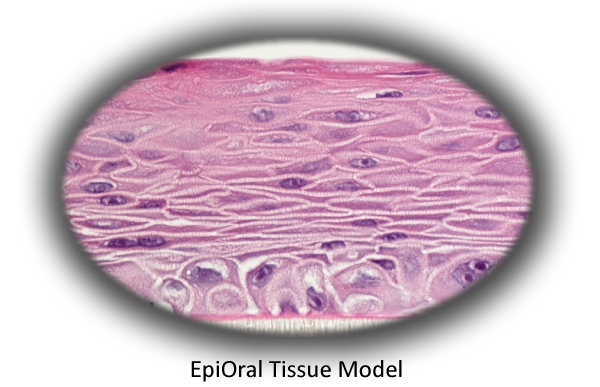
MatTek's EpiOral tissues consist of normal, human-derived epithelial cells. The tissues, which are cultured on specially prepared cell culture inserts using serum free medium, attain levels of differentiation on the cutting edge of in vitro cell culture technology. Morphologically, the tissue models closely parallel native buccal human tissues, thus providing a useful in vitro means to assess irritancy, disease and other basic oral biology phenomena. For more information on the EpiOral tissue model, click here.
The Method
Request the full protocol here.
-
Transfer tissues from agarose to assay medium
-
Incubate (37 ±1°C, 5 ±1% CO2, 95% RH) 1 h
-
Transfer tissues to receiver solution
-
Equilibrate tissues to permeation tempurature for 15 min
-
Apply permeant solution to apical surface of tissues
-
Collect, store and replace receiver solution every 30 - 60 min for the duration of the study
-
Determine average flux and calculate the permeability coefficient (Kp)
Technical References

670. IN VITRO EFFECTS OF ETHANOL AND MOUTHRINSE ON PERMEABILITY IN AN ORAL BUCCAL MUCOSAL TISSUE CONSTRUCT.
Koschier1, F., Kostrubsky2, V., Toole3, C., Gallo4, M.A. 1Johnson & Johnson Consumer Products Company, Morris Plains, NJ 07950, United States, 2Vistakon, Division of Johnson & Johnson Vision Care, Jacksonville, FL 32256, United States, 3CeeTox, Inc., Kalamazoo, MI 49008, United States, 4UMDNJ – Robert Wood Johnson Medical School, Piscataway, NJ 08854, United States. Food Chem Toxicol., 49, 10, 2534-9, (2011).
519. TRANSDERMAL AND BUCCAL DELIVERY OF METHYLXANTHINES THROUGH HUMAN TISSUE IN VITRO.
Thakur1, R.A., Michniak1, B.B., Meidan2, V.M. 1Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, Piscataway, NJ, 2Department of Pharmaceutical Sciences, University of Strathclyde, Glasgow, Scotland, UK. Drug Development and Industrial Pharmacy, 33, 513–521, (2007).
325. CHARACTERIZATION OF NEW BUCCAL AND GINGIVAL EPITHELIAL TISSUE MODELS.
Kubilus1, J., Breyfogle1, B., Sheasgreen1, J., Hayden1, P., and Wertz2, P., Dale3, B., Kimball3, J., and Klausner1, M. 1MatTek Corporation, Ashland, MA, 2University of Iowa, Iowa City, IA, 3University of Washington, Seattle, WA. J. Invest. Dermatol., 122, (3), A79, Abstract #474, (2004).
274. BARRIER FUNCTION COMPARISON BETWEEN IN VITRO SKIN, ORAL (BUCCAL), AND OCULAR TISSUE MODELS - IMPLICATIONS FOR DRUG DELIVERY STUDIES.
Klausner1, M., Kubilus1, J., Song2, Y., Michniak2, B., 1Department of Research and Development, MatTek, Ashland, MA, 2Department of Pharmacology and Physiology, UMDNJ-NJMS, Newark, NJ. Presented at the American Association of Pharmaceutical Scientists Meeting, November (2002).











www.MatTek.co.kr