Mucosal Irritation
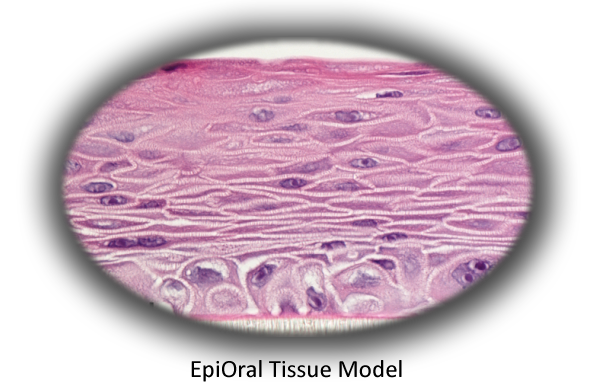
The Model
MatTek's EpiOral tissues consist of normal, human-derived epithelial cells. The tissues, which are cultured on specially prepared cell culture inserts using serum free medium, attain levels of differentiation on the cutting edge of in vitro cell culture technology. Morphologically, the tissue models closely parallel native buccal human tissues, thus providing a useful in vitro means to assess irritancy, disease and other basic oral biology phenomena. For more information on the EpiOral tissue model, click here.
The Method
Request the full protocol here.
-
Transfer tissues from agarose to assay medium
-
Incubate (37 ±1°C, 5 ±1% CO2, 95% RH) for 60 ±5 min
-
Transfer tissues to fresh assay medium
-
Dose tissues apically each with 40 μl TS (diluted 1:1 in ultrapureH2O), PC, or NC
-
Expose 20, 60 and 240 min (37 ±1°C, 5 ±1% CO2, 95% RH)
-
Stop exposure by rinsing in DPBS
-
Collect medium for cytokine analysis (optional)
-
Blot tissues and perform MTT assay
-
Read OD in a plate spectrophotometer at 550-570nm
The Endpoints
MTT Tissue Viability Assay
Cytokine Release (optional)
Technical References

507. DEVELOPMENT OF AN EPIORAL IN VITRO HUMAN TISSUE MODEL FOR ORAL IRRITANCY TESTING. Delves1, S.J., Faux1, S.P., Jai1, T.S., Kumaraveland1, T.S., Neilson2, L.R., and Meredith2, C. 1Toxicology Group, Advanced Technologies (Cambridge) Limited, 210 Cambridge Science Park, CB4 0WA, 2British American Tobacco, Group Research and Development, Southampton, SO15 8TL.
438. ORGANOTYPIC HUMAN ORAL TISSUE MODELS FOR TOXICOLOGICAL STUDIES. Klausner1, M., Ayehunie1, S., Breyfogle1, B.A., Wertz2, P.W., Bacca3, L., Kubilus1, J. 1MatTek Corporation, Ashland, MA 01721, United States, 2University of Iowa, Iowa City, IA 52242, United States, 3Procter and Gamble Company, Mason, OH 45040, United States. Toxicology in Vitro, 21, 938-949 (2007).
379. EPIORAL(ORL-100) AND EPIGINGIVAL (GIN-100) TISSUE MODELS FOR ORAL IRRITATION STUDIES. Breyfogle1,B., Kubilus1, J., Dale2, B., Wertz3, P. 1MatTek Corporation, Ashland, MA, USA, 2University of Washington, Seattle, WA, USA, 3University of Iowa, Iowa City, IA, USA. Presented at 5th World Congress, Berlin, Germany, August, (2005).
360. NOVEL IN VITRO METHODOLOGY EVALUATING POTENTIAL MUCOSAL IRRITATION OF ORAL CARE FORMULATIONS. Bacca, L.A., Jewell-Motz, E. A. Procter & Gamble Co., Mason, Ohio. Presented at the International Association of Dental Research Annual Meeting, Baltimore, MD, March 9-12, (2005).











www.MatTek.co.kr