EpiOcular MTT ET-50 (Dilution)
The Model
MatTek’s EpiOcular system consists of normal, human-derived epidermal keratinocytes which have been cultured to form a stratified, squamous epithelium similar to that found in the cornea. Cultured on specially prepared cell culture inserts using serum-free culture medium, the cells differentiate to form a multi-layered structure which closely parallels the corneal epithelium. For more information on the EpiOcular tissue model, click here.
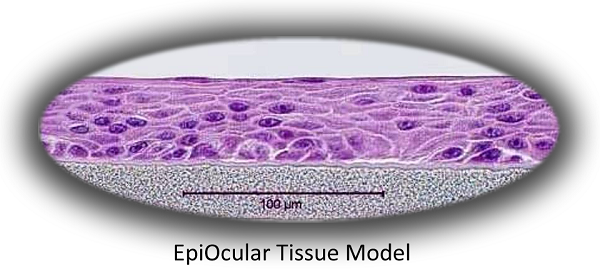
The Method
Request the full protocol here.
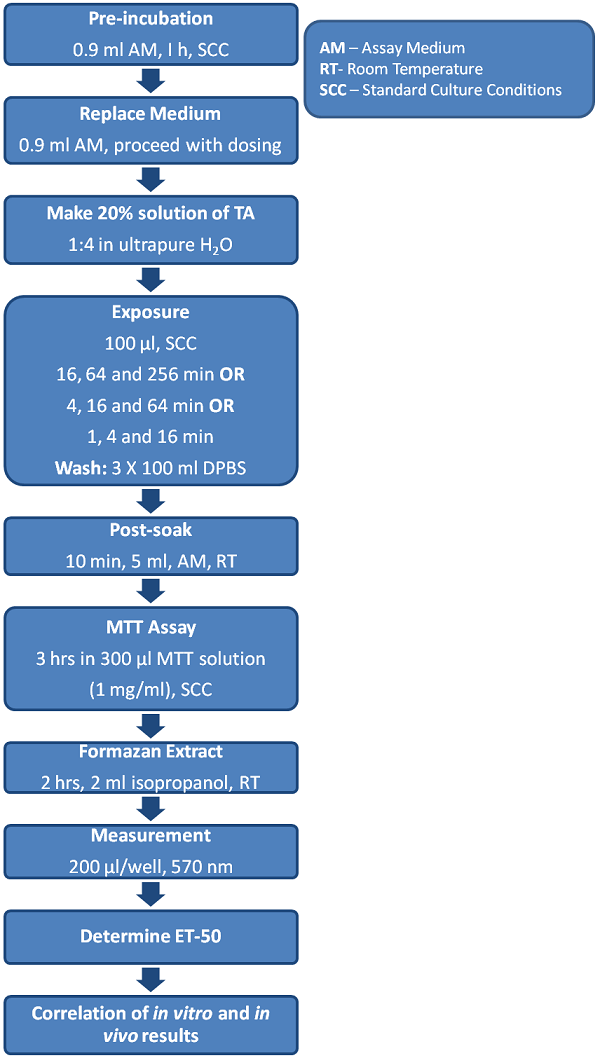
The Endpoints
MTT Tissue Viability Assay
Technical References

506. THE UTILIZATION OF THE EPIOCULAR HUMAN TISSUE MODEL TO ASSESS AND COMPARE THE IRRITATION POTENTIAL OF MULTIPLE SURFACTANT SYSTEMS USED IN SHAMPOOS AND FACIAL CLEANSERS.
Vavilikolanu1, P., Lazaro1, C., Mun2, G., Hilberer2, A., Hyder2, M., Raabe2, H., and Curren2, R. 1Alberto-Culver Company, Melrose Park, IL, USA 2Institute for In Vitro Sciences, Inc., Gaithersburg, MD, USA. Presented at the 47th Annual Society of Toxicology Meeting Seattle, WA, (2008). The Toxicologist, 102, 1, 66 (2008).
442. REGULATORY REQUIREMENTS FOR IN VITRO SYSTEMS TO MEET PERFORMANCE STANDARDS OVER TIME AS WELL AS DURING VALIDATION.
Klausner, M., Kubilus, J., Ayehunie, S., Hayden, P., Chua, G., Sheasgreen, J. MatTek Corporation, Ashland, MA 01721. Society of Toxicology 46th Annual Meeting, Charlotte, NC, Abstract 1524, (2007). The Toxicologist, 96, 1, 315 (2007).
402. DEVELOPMENT OF IMPROVED EPIOCULAR™ TEST PROTOCOLS FOR SCREENING OF SEVERE OCULAR IRRITANTS.
Jackson, G.R. Jr., Hayden, P.J., Kubilus, J., Kaluzhny, Y., Klausner, M. MatTek Corporation, Ashland, MA, USA. Presented at the 45th Society of Toxicology Meeting, San Diego CA, March 5-9, (2006). The Toxicologist, 90, 1, 326 (2006).
356. EPIOCULAR HUMAN CELL CONSTRUCT: TISSUE VIABILITY AND HISTOLOGICAL CHANGES FOLLOWING EXPOSURE TO SURFACTANTS.
Blazka1, M., Diaco2, M., Harbell2, J., Raabe2, H., Sizemore2, A., Wilt2, N., Bagley1, D. 1Colgate-Palmolive Co., Piscataway, NJ, USA; 2Institute for In Vitro Sciences, Inc., Gaithersburg, MD, USA. Presented at Society of Toxicology Annual Meeting, New Orleans, LA, March 6-10, 2005. The Toxicologist, 84, (1), Abstract # 2001, 409, (2005).
348. COMPARATIVE ASSESSMENT OF TWO EYE AREA COSMETIC FORMULATIONS THROUGH EVALUATION OF ALTERNATIVE EYE IRRITATION METHODS RELATIVE TO ENDPOINTS MEASURED IN A HUMAN CLINICAL SUB-ACUTE STUDY DESIGN.
Burdick2, J.D., Gao3, Y., Kanengiser3, B., Merrill1, J.C., Harbell1, J.W. 1Institute for In Vitro Sciences, Inc., Gaithersburg, MD; 2Bath & Body Works, Reynoldsburg, OH; 3Clinical Research Laboratories, Inc., Piscataway, NJ. Presented at Society of Toxicology Annual Meeting, Salt Lake City, Utah, March 9-13, (2003). The Toxicologist, 72, S1, 220 (2003).
287. EVALUATION OF MTT METABOLISM AS A MEANINGFUL INDICATOR OF VIABILTY IN HUMAN CORNEAL EPITHELIAL TISSUE MODELS.
Hines, M.D., Gettings, S.D. and Jones, B.C. Avon Products, Inc., Suffern, NY USA. The Toxicologist, 72, S1, 156 (2003).
147. THE EPIOCULAR PREDICTION MODEL: AN ACCURATE, REPRODUCIBLE IN VITRO MEANS OF ASSESSING OCULAR IRRITANCY POTENTIAL.
Klausner, M., Sennott, H.A., Breyfogle, B., Makwana, A., Kubilus, J. MatTek Corp., Ashland, MA. Toxicology In Vitro, 12, (4), 455-461 (1998).
139. PREDICTION OF OCULAR IRRITATION POTENTIAL OF AIR CARE PRODUCTS USING IN VITRO TOPICAL APPLICATION ASSAYS.
Manderfield1, C.E., Swanson1, J.E., Kennedy1, O., Lee2, M.K., Bauernschub2, M.A., Harbell2, J.W. 1S. C. Johnson & Son, Inc., Racine, WI, 2Microbiological Associates, Inc., Rockville, MD. Sponsored by R. D. Curren. The Toxicologist, 36 (1), 44, Soc. of Toxicol. (Reston, VA), Abstract #223, (1997).








www.MatTek.co.kr