EpiOcular Ultra-Mildness Test
The Model
MatTek’s EpiOcular system consists of normal, human-derived epidermal keratinocytes which have been cultured to form a stratified, squamous epithelium similar to that found in the cornea. Cultured on specially prepared cell culture inserts using serum-free culture medium, the cells differentiate to form a multi-layered structure which closely parallels the corneal epithelium. For more information on the EpiOcular tissue model, click here.
The Method
Request the full protocol here.
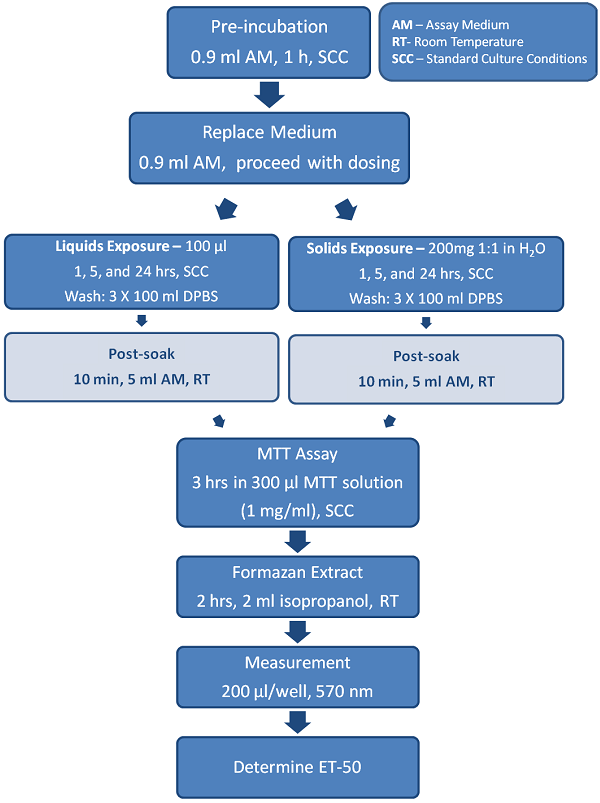
The Endpoints
MTT Tissue Viability Assay
Technical References

454. EXPERIENCES IN THE DEVELOPMENT AND UTILIZATION OF AN IN VITRO SAFETY TESTING PROGRAM FOR HAIR CONDITIONERS.
Vavilikolanu1, P., Lazaro1, C., Mun2, G., Hilberer2, A., Hyder2, M., Raabe2, H., and Curren2, R. 1Alberto-Culver Company, Melrose Park, IL, USA, 2Institute for In Vitro Sciences, Inc., Gaithersburg, MD, USA. Presented at the 6th World Congress on Alternatives and Animal Use in the Life Sciences, Tokyo, Japan August 21-25, (2007).
436. USE OF THE EPIOCULAR ASSAY FOR PRECLINICAL QUALIFICATION OF FORMULAS FOR HUMAN CLINICAL STUDIES.
Niranjan, P., Dang, A.H., January, B.G., Gomez, C., Harbell, J.W. Mary Kay, Inc., Dallas, TX, USA. Society of Toxicology 46th Annual Meeting, Charlotte, NC, (2007). The Toxicologist, 96, 1, 249 (2007).
402. DEVELOPMENT OF IMPROVED EPIOCULAR™ TEST PROTOCOLS FOR SCREENING OF SEVERE OCULAR IRRITANTS.
Jackson, G.R. Jr., Hayden, P.J., Kubilus, J., Kaluzhny, Y., Klausner, M. MatTek Corporation, Ashland, MA, USA. Presented at the 45th Society of Toxicology Meeting, San Diego CA, March 5-9, (2006). The Toxicologist, 90, 1, 326 (2006).
356. EPIOCULAR HUMAN CELL CONSTRUCT: TISSUE VIABILITY AND HISTOLOGICAL CHANGES FOLLOWING EXPOSURE TO SURFACTANTS.
Blazka1, M., Diaco2, M., Harbell2, J., Raabe2, H., Sizemore2, A., Wilt2, N., Bagley1, D. 1Colgate-Palmolive Co., Piscataway, NJ, USA; 2Institute for In Vitro Sciences, Inc., Gaithersburg, MD, USA. Presented at Society of Toxicology Annual Meeting, New Orleans, LA, March 6-10, 2005. The Toxicologist, 84, (1), Abstract # 2001, 409, (2005).
191. THE EPIOCULAR TISSUE MODEL: IN VIVO VERSUS IN VITRO DRAIZE SCORES FOR CONSUMER PRODUCTS.
Klausner1, M., Osborn1, M., Bellavance1, K., Breyfogle1, B., Kubilus1, J., Cerven2, D.R., DeGeorge2, G. 1MatTek Corp., Ashland, MA, 2MB Research Laboratories, Spinnerstown, PA Toxicological Sciences, 54, (1), 188 Abstr. #884 (2000).
167. THE EPIOCULAR PREDICTION MODEL - A REPRODUCIBLE IN VITRO MEANS OF ASSESSING OCULAR IRRITANCY POTENTIAL.
Klausner, M., Sennott, H.A., Breyfogle, B., Makwana, A., Kubilus, J. MatTek Corp., Ashland, MA, USA. The Toxicologist, 48 (1-S), 336, Soc. of Toxicology (Reston, VA), Abstract #1583, (1999).
142. EPIOCULAR PREDICTION MODEL. A REPRODUCIBLE IN VITRO TISSUE CULTURE MEANS OF PREDICTING DRAIZE SCORES.
Kubilus, J., Sennott, H., Makwana, A., Klausner, M. MatTek Corp., Ashland, MA. The Toxicologist, 36 (1), 44, Soc. of Toxicol. (Reston, VA), Abstract #222, (1997).








www.MatTek.co.kr