Ocular Irritation
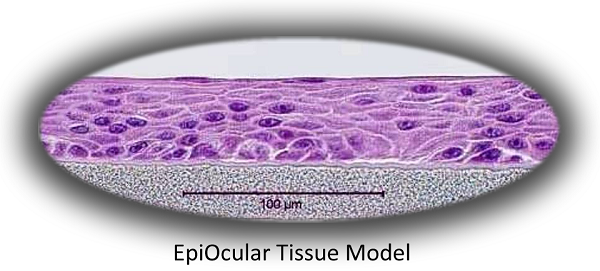
The Model
MatTek’s EpiOcular system consists of normal, human-derived epidermal keratinocytes which have been cultured to form a stratified, squamous epithelium similar to that found in the cornea. Cultured on specially prepared cell culture inserts using serum-free culture medium, the cells differentiate to form a multi-layered structure which closely parallels the corneal epithelium. For more information on the EpiOcular tissue model, click here.
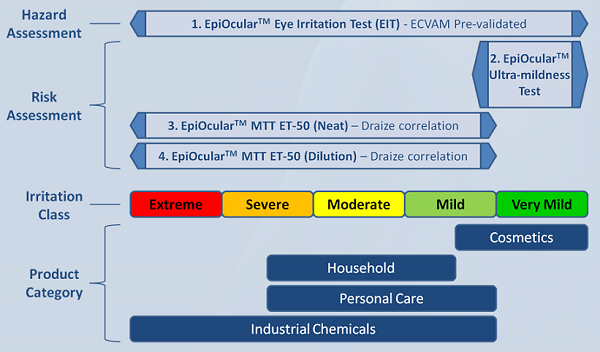
MatTek offers several ocular irritation assays based on the EpiOcularTM tissue model.
The Assays
1. EpiOcularTM Eye Irritation Test (EIT)
The EpiOcularTM EIT is an in vitro hazard assessment assay that has the ability to differentiate materials that are ocular non-irritants from materials that ocular irritants. The EIT is applicable to a broad range of organic and inorganic chemicals. Learn more.
2. EpiOcularTM "Sub-Draize" Ultra-mildness Test
The EpiOcularTM Ultra-mildness Test is an in vitro risk assessment assay routinely utilized for mild materials for which the Draize test is insensitive. This highly reproducible assay allows for quanifiable discrimination among mild, milder and mildest product formulations. Learn more.
3. EpiOcularTM MTT ET-50 (Neat)
This in vitro risk assessment assay is based on the effective time at which a material (applied neat) causes a 50% reduction in tissue viability (ET-50). Based on the ET-50, the test article is categorized into one of 4 classifications ranging from non-irritating to severe/extreme which correspond to groupings of Rabbit Draize Eye Scores (MMAS). Learn more.
4. EpiOcularTM MTT ET-50 (Dilution)
Similar to the MTT ET-50 Neat method, this in vitro risk assessment assay is based on the effective time at which a material (diluted to 20% in water) causes a 50% reduction in tissue viability (ET-50). Based on the ET-50, the test article is categorized into one of 4 classifications ranging from non-irritating to severe/extreme which correspond to groupings of Rabbit Draize Eye Scores (MMAS). This method is recommended for surfactant based solutions and is applicable to water-soluble materials with a specific gravity of > 0.95. Learn more.
Technical References

729. COSMETICS EUROPE MULTI-LABORATORY PRE-VALIDATION OF THE EPIOCULAR™ RECONSTITUTED HUMAN TISSUE TEST METHOD FOR THE PREDICTION OF EYE IRRITATION.
Pfannenbecker1, U., Bessou-Touya2, S., Faller3, C., Harbell4, J., Jacob5, T., Raabe6, H., Tailhardat7, M., Alépée8, N., De Smedt9, A., De Wever10, B., Jones11, P., Kaluzhny12, Y., Le Varlet13, B., McNamee14, P., Marrec-Fairley15, M., Van Goethem9, F. 1Beiersdorf AG, Hamburg, Germany. 2Laboratoire Pierre Fabre, Castres, France. 3Procter & Gamble/Cosmital, Marly, Switzerland. 4Mary Kay Inc., Dallas, TX, USA. 5Avon Products, Inc., Suffern, NY, USA. 6Institute for In Vitro Sciences, Inc., Gaithersburg, MD, USA. 7LVMH Recherche, St. Jean De Braye Cedex, France. 8L’Oréal Research & Innovation, Aulnay Sous Bois, France. 9Janssen Research & Development, Beerse, Belgium. 10Henkel AG & Co. KGaA, Düsseldorf, Germany. 11Safety and Environmental Assurance Centre, Unilever, Sharnbrook, UK. 12MatTek Corporation, Ashland, MA, USA. 13Consultant, Links Ingénierie, Paris, France. 14The Procter & Gamble Company, Egham, Surrey, UK. 15Colipa, Brussels, Belgium. Toxicology in Vitro, 27, 619–626, (2013).
706. PREDICTING EYE IRRITATION OF AGROCHEMICAL FORMULATIONS ACCORDING TO DIFFERENT CLASSIFICATION SCHEMES BY IN VITRO METHODS (BOVINE CORNEAL OPACITY AND PERMEABILITY AND EPIOCULAR EYE IRRITATION TEST).
Kolle1, S.N. Buechse2, A., Knieriem3, T., Mayer3, W., Rey Moreno1, M-C., van Cott4, A., van Ravenzwaay1, B., and Landsiedel1, R. 1BASF SE, Experimental Toxicology and Ecology, Ludwigshafen, Germany, 2BASF SE, Scientific Computing, Ludwigshafen, Germany, 3BASF SE, Agricultural Products – Formulation Development, Ludwigshafen, Germany, 4BASF Cooperation, Agricultural Products – Global Product Safety & Registration, Raleigh, NC, USA. EuroTox 2012.
688. UPDATE OF THE COLIPA EYE IRRITATION TASK FORCE STRATEGY AND PROGRAMME FOR DEVELOPMENT OF IN VITRO METHODS.
Alépée1, N., Bessou-Touya2, S., De Smedt3, A., De Wever4, B., Jones5, P., McNamee6, P., Marrec-Fairley7, M., Pfannenbecker8, U., Tailhardat9, M., Van Goethem3, F. 1L’Oréal, Aulnay Sous Bois Cedex, France, 2Laboratoire Pierre Fabre, Castres, France, 3Johnson & Johnson Pharmaceutical Research & Development, Beerse, Belgium, 4Henkel, Düsseldorf, Germany, 5Safety and Environmental Assurance Centre, Unilever, Sharnbrook, UK, 6The Procter & Gamble Company, Egham, Surrey, UK, 7Colipa, Brussels, Belgium, 8Beiersdorf, Hamburg, Germany, 9LVMH Recherche, France.
681. PROSPECTIVE VALIDATION STUDY OF RECONSTRUCTED HUMAN TISSUE MODELS FOR EYE IRRITATION TESTING.
McNamee1,2, P., Alépée3, N., Barroso4, J., Cole4, T., de Jong-Rubingh5, C., Eskes6, C., Freeman7, S.J., Kirmizidis4, G., Lammers8, J., Pfannenbecker9, U. and Zuang4, V. 1The Procter & Gamble Company, U.K.,2COLIPA, Belgium; 3L’Oréal, France; 4ECVAM, Institute for Health & Consumer Protection, European Commission Joint Research Centre, Italy; 5TNO, The Netherlands; 6Services & Consultation on Alternative Methods (SeCAM), Agno, Switzerland; 7Farino Consulting, UK; 8TNO Triskelion, The Netherlands; 9Beiersdorf, Germany. Presented at 8th World Congress on Alternatives and Animal Use, Montreal, Canada, 2011.
673. IN-HOUSE VALIDATION OF THE EPIOCULAR™ EYE IRRITATION TEST AND ITS COMBINATION WITH THE BOVINE CORNEAL OPACITY AND PERMEABILITY TEST FOR THE ASSESSMENT OF OCULAR IRRITATION.
Kolle1, S.N., Kandárová2, H., Wareing1, B., van Ravenzwaay1, B., and Landsiedel1, R. 1BASF SE, Experimental Toxicology and Ecology, Ludwigshafen, Germany; 2MatTek In Vitro Life Science Laboratories, Bratislava, Slovakia. ATLA, 39, 365–387, (2011). ATLA, 39, 365–387, (2011).
653. DEVELOPMENT OF THE EPIOCULAR™ EYE IRRITATION TEST FOR HAZARD IDENTIFICATION AND LABELLING OF EYE IRRITATING CHEMICALS IN RESPONSE TO THE REQUIREMENTS OF THE EU COSMETICS DIRECTIVE AND REACH LEGISLATION.
Kaluzhny1, Y., Kandárová2, H., Hayden1, P., Kubilus1, J., d’Argembeau-Thornton1, L., and Klausner1, M. 1MatTek Corporation, Ashland, MA, USA; 2MatTek In Vitro Life Science Laboratories, Bratislava, Slovakia. ATLA, 39, 339-364, (2011).
615. COLGATE-PALMOLIVE'S PROGRAM TO VALIDATE THE EPIOCULAR™ HUMAN TISSUE CONSTRUCT MODEL.
Rios-Blanco, M., Blazka, M. Colgate-Palmolive. Presented by Colgate at Paris, June 2003.
579. IN VITRO APPROACHES FOR PREDICTING IRRITATION POTENTIAL OF TOPICAL EXPOSURES TO THE SKIN AND EYES.
Harbell, J.W. Mary Kay, Dallas, Texas.
506. THE UTILIZATION OF THE EPIOCULAR HUMAN TISSUE MODEL TO ASSESS AND COMPARE THE IRRITATION POTENTIAL OF MULTIPLE SURFACTANT SYSTEMS USED IN SHAMPOOS AND FACIAL CLEANSERS.
Vavilikolanu1, P., Lazaro1, C., Mun2, G., Hilberer2, A., Hyder2, M., Raabe2, H., and Curren2, R. 1Alberto-Culver Company, Melrose Park, IL, USA 2Institute for In Vitro Sciences, Inc., Gaithersburg, MD, USA. Presented at the 47th Annual Society of Toxicology Meeting Seattle, WA, (2008). The Toxicologist, 102, 1, 66 (2008).








www.MatTek.co.kr