Skin Hydration ( EpiDermFT™ )
APPLICATION NOTE
In Vitro Evaluation of Cosmetic Formulations and Moisturizers
for Skin Hydration using EpiDermTM or EpiDermFTTM
Objective
To evaluate skin hydration following treatment with topically-applied cosmetic formulations or moisturizers by measuring the electrical impedance within the EpiDerm in vitro human skin model.
Methods
* EpiDerm tissues (Figure 1) were produced in the MatTek Corporation GMP tissue production facility .
* 25μl of each formulation was applied topically to the EpiDerm tissues for 4 hrs.
* After treatment, EpiDerm tissues were gently rinsed.
* Skin hydration was measured using the DPM 9003 Nova Meter (Nova Technologies).
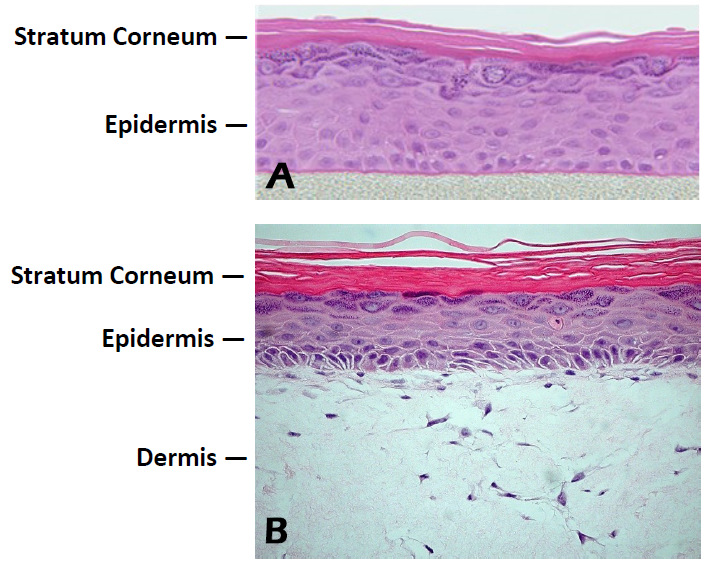
Figure 1. Histology of EpiDerm and EpiDermFT. H&E stained cross-section showing that the tissue morphology of EpiDerm
(A) and EpiDermFT (B) closely parallels that of normal human skin. The epidermis contains basal, spinous, granular and stratum corneum layers and the dermis contains viable fibroblasts (400x).
Results
Skin hydration levels were significantly higher in EpiDerm tissues treated with Formulation 1, Formulation 2 or Formulation 3 compared to the untreated controls (Figure 2).
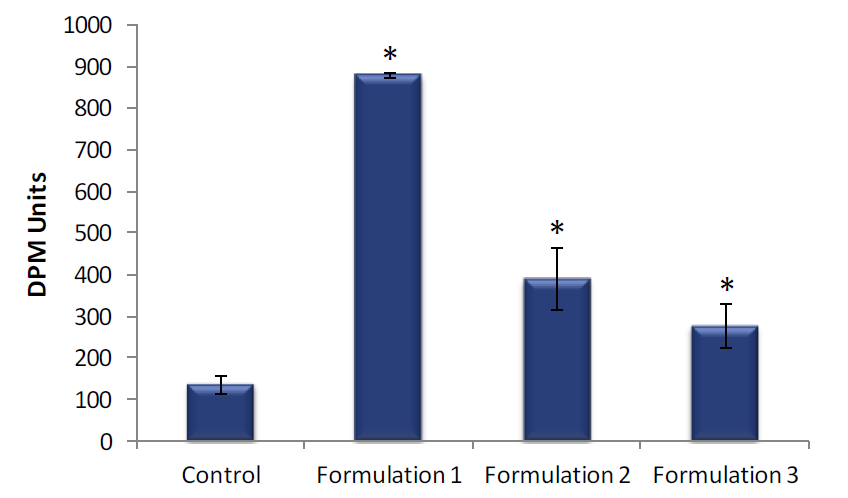
Figure 2. Skin hydration of EpiDerm. Skin hydration of EpiDerm was measured following a 4 hour topical exposure to Formulation 1, Formulation 2 or Formulation 3 and compared to untreated controls. Data are presented as individual experimental replicates (n=4). Skin hydration values that are significantly different from untreated control using a Student’s t-test (P ≤ 0.01) are indicated by an asterisk (*).
Conclusion
Assessment of skin hydration following treatment with topically-applied cosmetic formulations and moisturizers in the EpiDerm and EpiDermFT in vitro human skin models can be used in efficacy and claims substantiation studies.
Additional applications for the EpiDerm, MelanoDerm and EpiDermFT tissue models include Anti-aging, Skin Whitening, and UV Protection.
The Model
MatTek's EpiDermFT system consists of normal, human-derived epidermal keratinocytes and normal, human-derived dermal fibroblasts which have been cultured to form a multilayered, highly differentiated model of the human dermis and epidermis. Cultured on specially prepared cell culture inserts using serum-free culture medium, EpiDermFT attains levels of differentiation at the cutting edge of in vitro skin technology. For more information on the EpiDermFT tissue model, click here.
Technical References

634. UNDERSTANDING METABOLIC PATHWAYS FOR SKIN ANTI-AGING.
Osborne, R., Mullins, L.A., and Jarrold, B.B. P&G Beauty & Grooming, The Procter & Gamble Company, Miami Valley Innovation Center, Cincinnati, Ohio USA. J. of Drugs in Dermatol, 8, 7(Supplement), (2009).
518. IN VITRO SKIN BIOMARKER RESPONSES TO A NEW ANTI-AGING PEPTIDE, PAL-KT.
Osborne1, R., Mullins1, L.A., Jarrold1, B.B., Binder1, R.L., Mondon2, P., Lintner2, K. 1P&G Beauty & Grooming and Global Biotechnology, Cincinnati, Ohio USA. 2Sederma, France.
510. TOPICAL N-ACETYL GLUCOSAMINE AND NIACINAMIDE INCREASE HYALURONAN IN VITRO.
Osborne, R., Mullins, L.A., and Robinson, L.R. The Procter & Gamble Company, Cincinnati, Ohio USA.
488. STIMULATION OF EPIDERMAL HYALURONAN BY YEAST FERMENT.
Osborne1, R., Hakozaki2, T., Jarrold1, B., and Mullins1, L. 1Procter & Gamble Beauty, Cincinnati, Ohio, USA and 2Kobe, Japan.






www.MatTek.co.kr