Transdermal Drug Delivery ( EpiDerm )
The Model
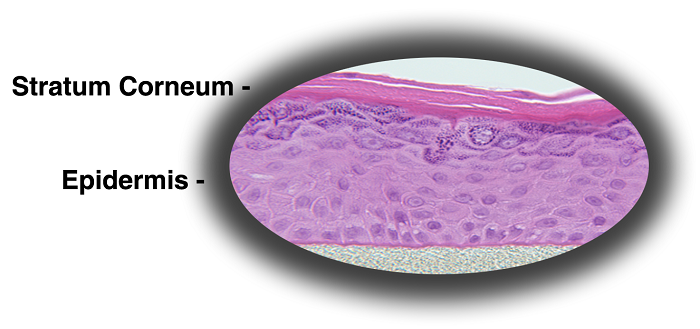
MatTek's patented EpiDerm™ System consists of normal, human-derived epidermal keratinocytes (NHEK) which have been cultured to form a multilayered, highly differentiated model of the human epidermis. These "ready-to-use" tissues, also known generically as Normal Human-3D (NHu-3D), are cultured on specially prepared cell culture inserts using serum free medium, attain levels of differentiation on the cutting edge of in vitro skin technology. Ultrastructurally, the EpiDerm Skin Model closely parallels human skin, thus providing a useful in vitro means to assess dermal irritancy and toxicology. For more information on the EpiDerm model, click here.
The Method
프로토콜 요청은 이곳으로.
-
Transfer tissues from agarose to assay medium
-
Incubate (37 ±1°C, 5 ±1% CO2, 95% RH) overnight (18 - 24 hrs)
- Transfer tissues to receiver solution
-
Equilibrate tissues to permeation tempurature for 15 min
- Apply permeant solution to apical surface of tissues
-
Collect, store and replace receiver solution every 30 - 60 min for the duration of the study
- Determine average flux and calculate the permeability coefficient (Kp)
Technical References

573. INHIBITION OF CRYSTALLIZATION IN DRUG-IN-ADHESIVE-TYPE TRANSDERMAL PATCHES. Jain, P., Banga, A.J. Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, 3001 Mercer University Drive, Mercer University, Atlanta, GA 30341-4155, United States. Inter. J. of Pharmaceutics, 394, 68–74, 2010.
517. DERMAL PENETRATION AND METABOLISM OF p-AMINOPHENOL AND p-PHENYLENEDIAMINE: APPLICATION OF THE EPIDERM HUMAN RECONSTRUCTED EPIDERMIS MODEL. Hua, T., Baileya, R.E., Morralla, S.W., Aardemaa, M.J., Stanleyb, L.A., Skarec, J.A. aThe Procter & Gamble Company, Miami Valley Innovation Center, Cincinnati, OH, 45253, US. bConsultant in Investigative Toxicology, P.O. Box 29225, St. Andrews, Fife, KY16 9WS, UK. cThe Procter & Gamble Company, Sharon Woods Technical Center, Cincinnati, OH, 45241, US. Toxicology Letters, 188, 119–129, 2009.
274. BARRIER FUNCTION COMPARISON BETWEEN IN VITRO SKIN, ORAL (BUCCAL), AND OCULAR TISSUE MODELS - IMPLICATIONS FOR DRUG DELIVERY STUDIES. Klausner1, M., Kubilus1, J., Song2, Y., Michniak2, B., 1Department of Research and Development, MatTek, Ashland, MA, 2Department of Pharmacology and Physiology, UMDNJ-NJMS, Newark, NJ. Presented at the American Association of Pharmaceutical Scientists Meeting, November (2002).








www.MatTek.co.kr