Dermal Genotoxicity ( EpiDerm )
The Model
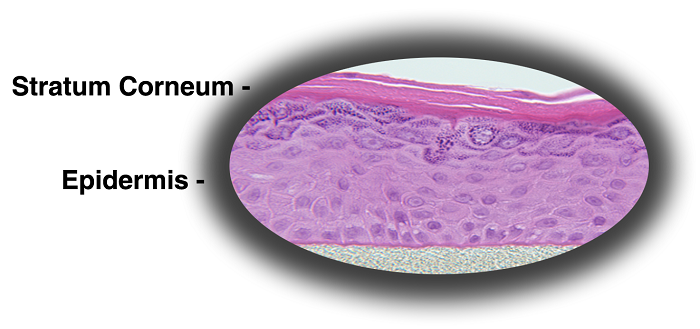
MatTek's patented EpiDerm™ System consists of normal, human-derived epidermal keratinocytes (NHEK) which have been cultured to form a multilayered, highly differentiated model of the human epidermis. These "ready-to-use" tissues, also known generically as Normal Human-3D (NHu-3D), are cultured on specially prepared cell culture inserts using serum free medium, attain levels of differentiation on the cutting edge of in vitro skin technology. Ultrastructurally, the EpiDerm Skin Model closely parallels human skin, thus providing a useful in vitro means to assess dermal irritancy and toxicology. For more information on the EpiDerm model, click here.
Technical References

730. METABOLICALLY COMPETENT HUMAN SKIN MODELS: ACTIVATION AND GENOTOXICITY OF BENZO[A]PYRENE. Brinkmann1, J., Stolpmann1, K., Trappe1, S., Otter1, T., Genkinger1, D., Bock2, U., Liebsch3, M., Henkler1, F., Hutzler1, C., and Luch1,3, A. 1German Federal Institute for Risk Assessment (BfR), Department of Product Safety, 10589 Berlin, Germany; 2Across Barriers GmbH, 66123 Saarbrücken, Germany; and 3German Federal Institute for Risk Assessment (BfR), Department of Experimental Toxicology and Center for Alternatives to Animal Testing, 12277 Berlin, Germany. Toxicological Sciences, 131(2), 351–359 2013.
687. COLIPA VALIDATION OF THE RECONSTRUCTED HUMAN SKIN MICRONUCLEUS ASSAY (RSMN): FURTHER PRE-VALIDATION STUDIES AND INVESTIGATIONS IN INCREASING TIME EFFICIENCY. Fautz1, R., Quedraogo2, G., Reisinger3, K., Aardema4, M., Barnett5, B., Faquet2, B., Mun6, G., Dahl6, E., Curren6, R., Hewitt7, N. and Pfuhler5, S. 1KPSS-Kao Professional Salon Services, Darmstadt, Germany; 2L’Oreal Life Sciences Research, Aulnay sous Bois, France; 3Henkel AG & Co KGaA, Duesseldorf, Germany; 4Bioreliance, Rockville, MD, USA, 5The Procter & Gamble Co., Cincinnati, OH, USA, 6Institute for In Vitro Sciences, Inc., Gaithersburg, MD, USA; 7Erzhausen, Germany.
686. THE USE OF 3D SKIN MODELS IN THE MICRONUCLEUS AND COMET ASSAYS: FURTHER PRE-VALIDATION STUDIES AND INVESTIGATIONS INTO INCREASING THROUGHPUT. Pfuhler1, S., Quedraogo2, G., Reisinger3, K., Krul4, C., Curren5, R., Aardema6, M., Fautz7, R., Barnett1, B., Downs1, T., Reuss4, A., Faquet2, B., Mun5, G., Hewitt8, N. 1The Procter & Gamble Co., Cincinnati, OH, USA; 2L’Oreal Life Sciences Research, Aulnay sous Bois, France; 3Henkel AG & Co KGaA, Duesseldorf, Germany; 4TNO, The Netherlands; 5Institute for In Vitro Sciences, Inc., Gaithersburg, MD, USA; 6Marilyn Aardema Consulting, LLC, USA; 7KPSS-Kao Professional Salon Services, Darmstadt, Germany; 8Erzhausen, Germany.
645. THE RECONSTRUCTED SKIN COMET ASSAY SHOWS GOOD INTRA- AND INTER-LABORATORY REPRODUCIBILITY: RESULTS FROM THREE LABORATORIES. Downs1, T., Reus2,A., Reisinger3, K., Krul2, C., and Pfuhler1, S. 1Procter & Gamble Co., Cincinnati, OH, USA, 2TNO Quality of Life, Zeist, The Netherlands, 3Henkell AG & Co KgaA, Dusseldorf, Germany. Presented at SOT 2011, Abstract #341
633. IN VITRO GENOTOXICITY TESTING USING THE MICRONUCLEUS ASSAY IN CELL LINES, HUMAN LYMPHOCYTES AND 3D HUMAN SKIN MODELS. Kirsch-Volders1, M., Decordier1, I., Elhajouji1, A., Plas2, G., Aardema2, M.J. and Fenech3, M. Laboratorium voor Cellulaire Genetica, Vrije Universiteit Brussel, Pleinlaan 2, 1050 Brussels, Belgium, 1Novartis Institutes for Biomedical Research, Translational Sciences, Preclinical Safety, Genetic Toxicology and Safety Pharmacology, CH-4002 Basel, Switzerland, 2Marilyn Aardema Consulting, LLC, 5315 Oakbrook Dr., Fairfield, OH 45014, USA and 3CSIRO Food and Nutritional Sciences, Nutritional Genomics, Gate 13, Kintore Avenue, PO Box 10041, Adelaide BC, SA 5000, Australia. Mutagenesis, 26, (1), 177-184 (2011).








www.MatTek.co.kr