Skin Hydration ( EpiDerm )
The Model
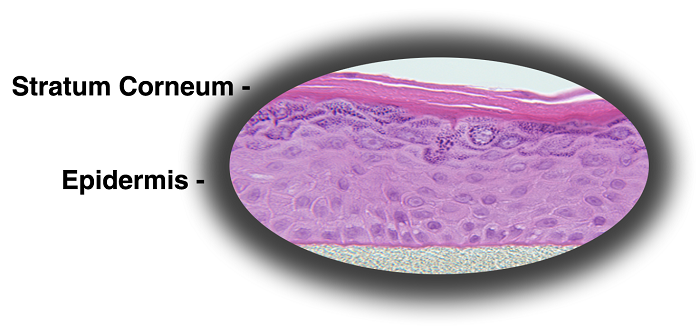
MatTek's patented EpiDerm™ System consists of normal, human-derived epidermal keratinocytes (NHEK) which have been cultured to form a multilayered, highly differentiated model of the human epidermis. These "ready-to-use" tissues, also known generically as Normal Human-3D (NHu-3D), are cultured on specially prepared cell culture inserts using serum free medium, attain levels of differentiation on the cutting edge of in vitro skin technology. Ultrastructurally, the EpiDerm Skin Model closely parallels human skin, thus providing a useful in vitro means to assess dermal irritancy and toxicology. For more information on the EpiDerm model, click here.
In Vitro Evaluation of Cosmetic Formulations and Moisturizers
for Skin Hydration using EpiDermTM or EpiDermFTTM
Objective
To evaluate skin hydration following treatment with
topically-applied cosmetic formulations or moisturizers by
measuring the electrical impedance within the EpiDerm in
vitro human skin model.
Methods
* EpiDerm tissues (Figure 1) were produced in the
MatTek Corporation GMP tissue production facility .
* 25μl of each formulation was applied topically to the
EpiDerm tissues for 4 hrs.
* After treatment, EpiDerm tissues were gently rinsed.
* Skin hydration was measured using the DPM 9003
Nova Meter (Nova Technologies).
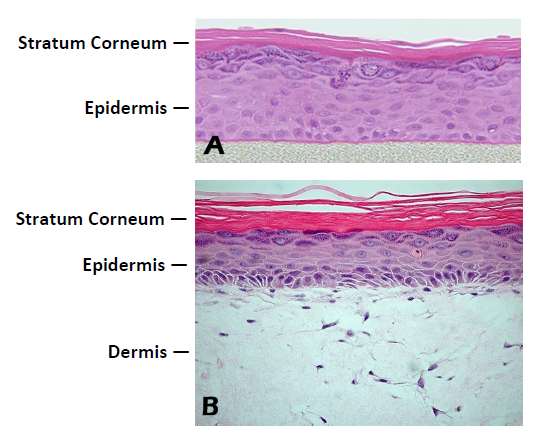
Figure 1. Histology of EpiDerm and EpiDermFT.
H&E stained cross-section showing that the tissue morphology of EpiDerm
(A) and EpiDermFT (B) closely parallels that of normal human skin. The epidermis contains basal, spinous, granular and stratum corneum layers and the dermis contains viable
fibroblasts (400x).
Results
Skin hydration levels were significantly higher in EpiDerm tissues treated with Formulation 1, Formulation 2 or Formulation 3 compared to the untreated controls
(Figure 2).
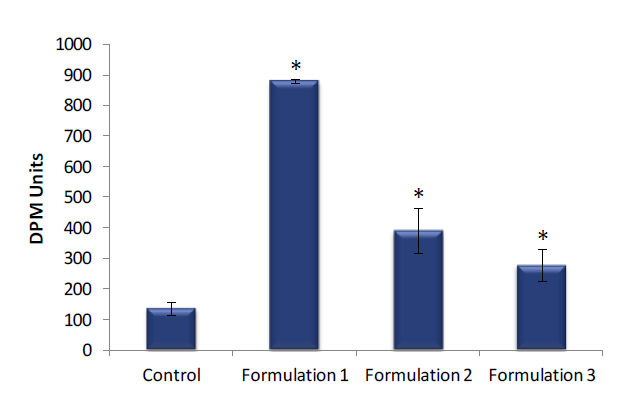
Figure 2. Skin hydration of EpiDerm. Skin hydration of EpiDerm was measured following a 4 hour topical exposure to Formulation 1, Formulation 2 or Formulation 3 and compared to untreated controls. Data are presented as individual experimental replicates (n=4). Skin hydration values that are
significantly different from untreated control using a Student’s t-test (P ≤ 0.01) are indicated by an asterisk (*).
Conclusion
Assessment of skin hydration following treatment with topically-applied cosmetic formulations and moisturizers in the EpiDerm and EpiDermFT in vitro human skin
models can be used in efficacy and claims substantiation studies.
Additional applications for the EpiDerm, MelanoDerm and EpiDermFT tissue models include Anti-aging, Skin Whitening, and UV Protection.
Technical References

336. ROLE OF TAURINE ACCUMULATION IN KERATINOCYTE HYDRATION.
Janeke*, G., Siefken, W., Carstensen, S., Springmann, G., Bleck**, O., Steinhart*, H., Höger**, P., Wittern, K.P., Wenck, H., Stäb, F., Sauermann, G., Schreiner, V., Doering, T. R&D cosmed, Beiersdorf AG, Hamburg, Germany; *Department of Food Chemistry and **Department of Dermatology, University of Hamburg, Germany. J. Invest. Dermatol., 121, (2), 354-361, (2003).
303. GENE EXPRESSION PROFILE OF TISSUE ENGINEERED SKIN SUBJECTED TO ACUTE BARRIER DISRUPTION.
Koria1, P., Brazeau2, D., Kirkwood3, K., Hayden4, P., Klausner4, M., Andreadis1, S. 1Department of Chemical Engineering, University at Buffalo, State University of New York, Amherst, NY, 2Department of Pharmaceutical Sciences, University at Buffalo, State University of New York, Amherst, NY, 3Department of Periodontics and Endodontics, University at Buffalo, State University of New York, Amhert, NY, 4MatTek Corportation, Ashland, MA. J. Investigative Dermatology, 121, (2), 368-382, (2003).
280. COMPARISON OF CUTANEOUS BIOAVAILABILITY OF COSMETIC PREPARATIONS CONTAINING CAFFEINE OR ALPHA-TOCOPHEROL APPLIED ON HUMAN SKIN MODELS OR HUMAN SKIN EX VIVO AT FINITE DOSES.
Dreher, F., Fouchard, F., Patouillet, C., Andrian, M., Simonnet, J., Benech-Kieffer, F. L'Oreal Research, Aulnay-sous-Bois, France. Skin Pharmacology and Applied Skin Physiology, 15, (Suppl 1), 40-58, (2002).
231. COMPARISON OF CUTANEOUS BIOAVAILABILIY OF COSMETIC PREPARATIONS CONTAINING CAFFEINE OR α-TOCOPHEROL APPLIED ON HUMAN SKIN MODELS OR HUMAN SKIN EX VIVO AT FINITE DOSES.
Dreher1, F., Fouchard1, F., Patouillet1, C., Andrian1, M., Simonnet2, J-T., Benech-Kieffer1, F. 1Life Sciences, L'OREAL advanced Research Laboratories, Aulnay-sous-BoisFrance, 2L'OREAL Applied Research, Chevilly-Larue France. Presented at Gordon Research Conference on "Barrier Function of Mammalian Skin", Bristol, USA, August 5-10, (2001).








www.MatTek.co.kr