In Vitro 3-D Tissue Model Basics
Assays based on MatTek "ready-to-use" in vitro 3-D, organotypic human tissue equivalents are well-documented and based on validated protocols. A basic overview of the production process and of a typical assay follows using the EpiDerm™ Model EPI-200 Kit as an example.
VIDEO Overview:
EpiDerm VIDEO Technical Article - Watch First 4 Minutes to Learn About MatTek's In Vitro 3-D Human Tissue Equivalents. Watch Entire Video for Detailed Instructions on How to Perform the ECVAM-Validated EpiDerm Skin Irritation Test (SIT) Protocol.
Tissue Production:
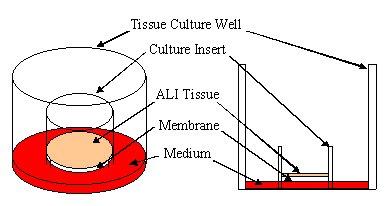
MatTek uses normal (non-transformed) donated human cells as the basis for all of its 3-D tissue equivalents. These cells are grown (cultured) in standard Millipore Millicell™ Single Well Tissue Culture Plate Inserts at the air liquid interface or ALI (see diagram).
 |
|
Diagram - Millicell Single Well Tissue Culture Plate Insert in a well of a multi-well tissue culture plate showing tissue at the Air-Liquid Interface (ALI). Apical (top) surface of tissue is exposed to air allowing for direct application of test articles. Click on small image above to see larger image. |
Note: Tissues can be ordered in other formats, including Corning Snapwell™ inserts and Millipore 24-well and 96-well plates.
At a specific point in this process, all liquid is removed from the apical (top) surface of the tissue. The 3-D tissues are then fed only through the basolateral (bottom) surface, which remains in contact with MatTek’s proprietary culture medium.
The process described above is initiated at MatTek after receipt of Customer orders and takes approximately 3 weeks to complete, depending on the tissue being grown.
A typical EpiDerm shipment (1 kit, EPI-200) contains 24 tissues, each tissue 9 mm in diameter and in its own culture plate insert, plus a small amount of medium. For shipment, each insert is placed in one well of a standard 24-well plate. Each well contains medium-infused agarose gel and the insert is seated firmly on this gel. Most tissues are shipped on Monday for delivery on Tuesday (USA) or Wednesday (Europe). Some overseas shipments are also initiated on a Friday for delivery on the following Monday or Tuesday.
 |
|
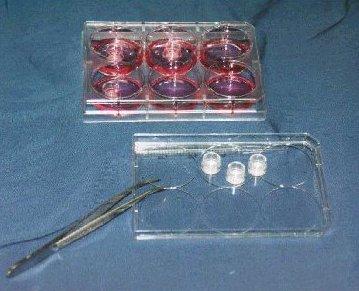
Shown: 6-well tissue culture plate with 6 single well tissue culture plate inserts containing EpiDerm (EPI-200),
1 insert in each of 3 wells, and 3 inserts sitting on plate cover. |
Description of a Typical Assay:
For testing, the top (apical) surface of each 3-D tissue is exposed to a precise amount of the sample of interest or a control substance while the tissue is fed with culture medium through the basolateral (bottom) surface (see photo). Samples and controls are typically run in duplicate or triplicate.
After the prescribed exposure period outlined in the protocol, the sample or control substance is rinsed from the tissue and the appropriate end-point assay is run.
Many of MatTek’s protocols call for the end point to be determined using the MTT ET-50 Tissue Viability Assay1, a quantitative, convenient method for evaluating a tissue's viability in response to external factors. (For EpiDerm, the correct MTT Kit to use is catalog number MTT-100.)
Changes in histology, cytokine release, and gene regulation can also be monitored. It is important to note, however, that data generated with these supplemental end points are often rendered suspect or misleading without the inclusion of time-matched positive and negative controls that determine tissue viability using the MTT assay.
Major lab equipment required to perform most MatTek in vitro 3-D human tissue equivalent assays include a standard CO2 incubator, a laminar flow hood, and a colorimetric (ELISA) plate reader capable of reading 96-well plates. Additional equipment and supplies used to perform these assays can be reviewed by reading a MatTek test protocol.
www.MatTek.co.kr